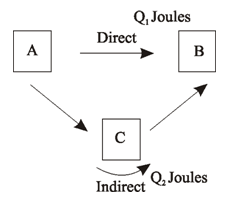
Hess’s law can be proved on the basis of first law of thermodynamics, according to which heat can neither be created nor destroyed. Let us suppose Q1 Joules is heat change when A changes to B directly and Q2 Joules is when A changes B indirectly via C. Let us suppose Q1 > Q2. Now if we move from A to B directly and then come back via C, then (Q1 – Q2) will be the heat evolved. This means that when we move from A to B and then back from B to A certain amount of heat is created which is against first law of thermodynamics. Thus we conclude, that Q1 cannot be more than nor less than Q2, but are equal, thus verifies Hess’s Law.
Hess law proof in long question
ReplyDeleteNice
ReplyDeleteSome times its a pain in the ass to read what blog owners wrote but this site is really user pleasant! . نموذج لائحة اعتراضية حيازة مخدرات
ReplyDelete