1. Heat of Formation
It is the quantity of heat evolved or absorbed (i.e. change in enthalpy). When one mole of the substance is formed from its constituent elements under given set of conditions. It is represented by DHf.
If the temperature and pressure are made 25o and 1 bar i.e. standard conditions. The heat of formation is then known as Standard Heat of Formation. Thus, standard heat of formation may be defined as, “the heat change accompanying the formation of 1 mole of substance in the standard state from its elements also taken in standard state.”
Example:
When 1 mole of CO2 (g) is formed from its element i.e. C(s) and O2(g) at standard state, 393.5 KJ of heat is evolved. Hence Standard Enthalpy of Formation of Gaseous CO2 is 393.5 KJ/mol. The enthalpies of formation of all free elements in their standard state are taken as zero. In case of allotropic modification, the standard enthalpy of stablest form is taken as zero. For example, in case of carbon, graphite has zero standard enthalpy of formation not that of diamond because it is less stable.
Standard Enthalpy of Reaction = | Standard enthalpy of formation of products | – Standard enthalpy of formation of reactants. |
2. Heat of Combustion
Heat of combustion may be defined as, “The amount of heat evolved or absorbed when one mole of the substance is completely burnt in air or oxygen.”
CH4 (g) + 2O2 (g) ------------> CO2 (g) + 2H2O (g) ; DH = – 890.4 KJ/Mol.
Calorific Values of foods and Fuels
“The calorific value of fuel or food is the amount of heat in calories or joules produced from the complete combustion of one gram of the fuel or the food.”
For example:
1 mole of CH4 liberates energy = 890.4 KJ.
i.e. 16 gm of CH4 liberates energy = 890.4 KJ
1 gm of CH4 liberates energy = 890.4/16 = 55.65 KJ
Thus, Calorific value of methane is 55.65 KJ.
3. Heat of Neutralization
Heat of neutralization may be defined as, “The amount of heat evolved when one equivalent of an acid is neutralized by one equivalent of a base in fairly dilute solution.”
For Example:
HCl (aq.) + NaOH (aq.) ------------> NaCl (aq.) + H2O ; DH = – 13.7 K cal.
It has been observed that heat of neutralization of a strong acid against a strong base is always constant (13.7 Kcal or 57 KJ per mole).
4. Heat of Solution
"It is the amount of heat evolved or absorbed when one mole of the solute is dissolved completely in excess of solvent.”
For Example:
NH4Cl (s) + H2O (l) ------------> NH4Cl (aq.) ; DH = + 3.90 K Cal
BaCl2 (s) + H2O (l) ------------> BaCl2 (aq.) ; DH = – 2.70 K Cal.
5. Heat of Dilution
“It is the change in enthalpy when 1 mole of a substance is diluted to such an extent that on further dilution no heat is evolved or absorbed.”
6. Heat of Hydration
Heat of hydration may be defined as, “The amount of heat evolved or absorbed when 1 mole of an anhydrous or a partly hydrated salt combined with the required number of moles of water to form a specific hydrate.”
For Example:
CuSO4 (s) + 5H2O (l) ------------> CuSO4 . 5H2O ; DH = – 18.69 K Cal.
7. Heat of Fusion
Heat of fusion may be defined as, “The heat evolved or absorbed when one mole of solid substance changes into its liquid state at its melting point.”
For Example:
8. Heat of Vapourisation
Heat of vapourisation may be defined as, “the heat evolved or absorbed when one mole of a liquid changes into its gaseous state at its boiling point.”
For Example:
9. Heat of Sublimation
Heat of sublimation may be defined as, “The heat evolved or absorbed when one mole of a solid changes directly into its vapour state at a given temperature below its melting point.”
Heat of sublimation may be defined as, “The heat evolved or absorbed when one mole of a solid changes directly into its vapour state at a given temperature below its melting point.”
For Example:
I2 (s) ------------> I2 (g) ; DH = 62.39 KJ/Mol.

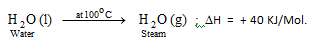
No comments:
Post a Comment