There are three common types of hybridization in compounds;
1. Tetrahedral or sp3 Hybridization
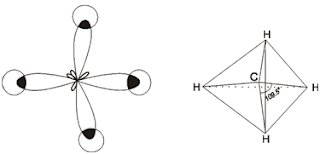
The mixing of one 's' and three 'p' orbitals to form four equivalent hybrid orbitals is called sp3 hybridization. Due to mutual repulsion of electrons in these four orbitals, sp3 hybrid orbitals try to keep themselves as far away as possible from each other. The arrangement in space that keeps them farthest apart is that of a tetrahedron. Thus, the four hybrid orbitals are directed towards the four corners of a regular tetrahedron, making an angle of 109.50 with each other. Because of their tetrahedral orientation, this hybridization is also called tetrahedral hybridization.
For Example:–
In methane, the central atom is carbon, with the electronic configuration 1s2, 2s2 2px12py1.
Carbon atom (At. No. 6)
Carbon atom in the ground state has only two unpaired electrons but in the excited state one 2s electron is promoted to vacant 2pz orbital. Thus there are four unpaired electrons, one each in 2s, 2px, 2py and 2pz orbitals. These four orbitals hybridize and form four equivalent sp3 hybrid orbitals directed towards the four corners of a regular tetrahedron with bond angle 109.5o.
Each sp3 hybrid orbital, overlaps 1s orbital of hydrogen atom and forms four C – H bonds in CH4 molecule. Thus each C–H bond involves sp3 – s overlap and methane molecule has tetrahedral structure with H – C – H bond angle 109.5o.
Formation of methane (CH4) molecule:
2. Triagonal or sp2 hybridization
The mixing of one s & two p orbitals to form three new equivalent hybrid orbitals to form three new equivalent hybrid orbitals is called sp2 hybridization. The three hybrid orbitals lie in one plane, making an angle of 1200 with each other to avoid mutual repulsion.
For example
Formation of ethylene (C2H2) molecule:
During the formation of ethylene molecule, each carbon atom undergoes sp2 hybridization.
In the excited state of carbon atom, out of the four orbitals, each containing an unpaired electron, only three orbitals i.e., one 2s and two 2p orbitals (2px and 2py) undergo sp2 hybridization. The resulting three hybrid orbitals are planar inclined at an angle of 120° and lie in xy – plane while the 2pz atomic orbital which has not taken part in hybridization, lies perpendicular to the hybrid orbitals.
One of the sp2 hybrid orbital of a carbon atom overlaps with one sp2 hybrid orbital of the other carbon atom by head on collision and forms σ bond between the two carbon atoms. The remaining two hybrid orbitals of each carbon atom overlap with 1s
orbitals of hydrogen atoms to form σ bonds. Thus, each carbon atom is left with one unhybridized 2pz orbital with lobes above and below the plane of hybrid orbitals. These two 2pz orbitals of the two carbon atoms overlap laterally and form a pi (π) bond between the two carbon atoms.
orbitals of hydrogen atoms to form σ bonds. Thus, each carbon atom is left with one unhybridized 2pz orbital with lobes above and below the plane of hybrid orbitals. These two 2pz orbitals of the two carbon atoms overlap laterally and form a pi (π) bond between the two carbon atoms.
Thus, in ethylene molecule, there is a double bond between the two carbon atoms, out of which one is σ bond involving sp2-sp2
overlap and the other is a π bond involving p-p overlap. In all, in the ethylene molecule, there are five σ bonds
and one π bond.
overlap and the other is a π bond involving p-p overlap. In all, in the ethylene molecule, there are five σ bonds
and one π bond.
3. Diagonal or sp Hybridization
The mixing of one s and one p – orbital to form two equivalent hybrid orbitals is called sp hybridization. The hybrid orbitals lie as far apart as possible from each other to minimize the forces of repulsion. Hence, they point in opposite directions, making an angle of 1800.
For example
Formation of acetylene (C2H2) molecule:
In acetylene molecule, each carbon atom undergoes sp hybridization.
In the excited state of carbon atom, there are four half filled orbitals. Out of these, two orbitals, i.e., 2s and 2px undergo sp hybridization and form two equivalent linear hybrid orbitals. The other two orbitals 2py and 2pz that have not taken part in hybridization remain at right angles to the hybrid orbitals. One of the hybrid orbitals of each carbon atom overlaps 1s orbital of H atom and form a σ bond. The remaining hybrid orbital of the two C atoms overlap to forms a σ bond between the two carbon atoms. Thus, each C atom is left with two unhybridized p orbitals (2py and 2pz) which are mutually perpendicular to H–C–C–H axis. These p orbitals overlap sideways and form two pi bonds between the two carbon atoms.
Thus, in acetylene molecule, there is a triple bond between the two carbon atoms, out of which one is σ bond involving sp-sp overlap and the other two are pi-bonds involving p-p overlap. In all, in the acetylene molecule, there are three σ bonds and two π bonds.









very helpful
ReplyDeleteThanks whoever has published this information....
ReplyDeleteThank you so much.
ReplyDeleteVery helpful... credit to the publisher of this informations.
ReplyDeleteThanks alot.Ive been helped greatly
ReplyDeletegot an idea...thanx dood!!!
ReplyDeleteTnxxxx
ReplyDeleteC2H2 same structure in two place??
ReplyDeleteWhat is sp3d hybridization
ReplyDeleteGreat reading yourr blog post
ReplyDeleteThis is a very clear explanation of hybridization.
ReplyDelete