Following are the factors which
influence the adsorption of gases by solids.
 In
order to understand the effect of pressure on adsorption of gas on some solid
we must keep in mind that physical adsorption is reversible in nature and is accompanied
by decrease in pressure. Therefore, it is expected that extent of adsorption
increases with increase in pressure and decrease in pressure causes desorption.
In
order to understand the effect of pressure on adsorption of gas on some solid
we must keep in mind that physical adsorption is reversible in nature and is accompanied
by decrease in pressure. Therefore, it is expected that extent of adsorption
increases with increase in pressure and decrease in pressure causes desorption.
and the corresponding
pressure (Ps)
is called Saturation Pressure. Such isotherms are obtained in cases where
adsorbing gases forms unimolecular layers on the surface of adsorbent and
adsorbing gas behaves ideally in vapour phase.
1. Surface
area
2. Nature
of gas
3. Temperature
4. Pressure
1. Surface Area
Adsorption being a surface
phenomenon, the extent of adsorption depends upon the surface area. Increase in
the surface area of the adsorbent, increases the total amount of gas adsorbed.
Thus finely divided metals (nickel, platinum) and porous substances (Charcoal,
silica gel) provides large surface area and are best solid adsorbents.
2. Nature of Gas
The amount of gas adsorbed by a solid depends
upon the nature of gas. In general, more easily liquefiable a gas is (i.e. higher its critical temperature), the
more readily will it be adsorbed. Thus 1gm of activated charcoal adsorbs 380 ml
of sulphur dioxide (critical temperature
157°C), 16 ml of methane (critical
temperature –83°C) and 4.5 ml of hydrogen (critical temperature –240°C).
This is valid for physical adsorption only.
Since Chemical
Adsorption is Specific in nature, it occurs only if the gas can form a chemical
bond with the solid.
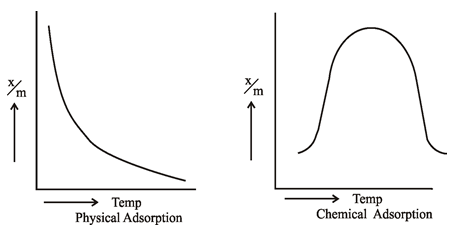
3. Tempreture
The process of Adsorption is an Exothermic
Reaction. Thus according to Le-chatlier’s Principle, the
magnitude of adsorption should increase with decrease in temperature. Infact it
is found to be so in case of physical adsorption because vanderwaal’s forces
are strong at low temperatures. However, the chemisorption first increases with
rise in temperature and then starts decreasing. The initial increase shows that
like chemical reactions, chemisorption also needs activation energy.
If
a plot is drawn between amount of gas adsorbed (x/m) and temperature at
constant equilibrium pressure, then curve obtained for physical adsorption
shows there is a regular decrease in adsorption with temperature rise. While
for chemisorption it first increases and then shows regular decrease. Such curves
are known as Adsorption Isobars.
4. Effect of Pressure
 In
order to understand the effect of pressure on adsorption of gas on some solid
we must keep in mind that physical adsorption is reversible in nature and is accompanied
by decrease in pressure. Therefore, it is expected that extent of adsorption
increases with increase in pressure and decrease in pressure causes desorption.
In
order to understand the effect of pressure on adsorption of gas on some solid
we must keep in mind that physical adsorption is reversible in nature and is accompanied
by decrease in pressure. Therefore, it is expected that extent of adsorption
increases with increase in pressure and decrease in pressure causes desorption.
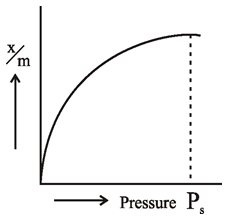
The
extent of adsorption is generally expressed as x/m where ‘m’ is mass of
adsorbent and ‘x’ is mass of adsorbate when equilibrium has attained. The graph
between extent of adsorption (x/m) and the pressure ‘P’
of gas at constant temperature is called Adsorption Isotherm.
This is a simple type of adsorption isotherm in which at equilibrium pressure Ps, reaches its maximum value and no more adsorption takes place even if the pressure is further increased. This state is also called SaturationState
This is a simple type of adsorption isotherm in which at equilibrium pressure Ps, reaches its maximum value and no more adsorption takes place even if the pressure is further increased. This state is also called SaturationState
i was reading the blog and i think that this information is going to help me a lot.
ReplyDeletewww.n8fan.net
haha chutiya
DeleteNice content. I love your blog because it's very informative. Keep posting more. Thank you.
ReplyDeleteWhiz
www.imarksweb.org
Can you tell me why multimolecular layer is formed at high pressure
ReplyDeletecan u tell me how surface defect influence the adsorption
ReplyDeleteyes my dear
DeleteThis comment has been removed by the author.
ReplyDeletenice info keep sharing
ReplyDeleteNice work
ReplyDeleteWhat a ans.
ReplyDeleteI m searching many sites but didn't get the right one,here I get the best.
Thanku for this ans
I agree
ReplyDeleteThank you
ReplyDeletebsdk blog hai yeh
ReplyDelete