Let us apply VSEPR theory to predict the shape of few molecules;
1. Boron triflouride (BF3)
In BF3 molecule, the Boron atom has atomic number 5 and has electronic configuration of 2, 3. Thus, Boron has 3 valence electrons. These electrons are shared mutually with three fluorine atoms to form three B–F bonds. So, in BF3, Boron has no lone pair over it.
No. of bond pairs = 3
No. of lone pairs = 0
Total no. electron pairs = bp + lp
= 3 + 0 = 3
So, according to VSEPR theory, BF3 has triangular planar shape. This is because it has 3 electron pairs.
2. Methane CH4
In CH4 molecule, the Carbon atom has atomic number 6 and has electronic configuration of 2, 4. Thus, Carbon has 4 valence electrons. These electrons are shared mutually with three hydrogen atoms to form four C–H bonds. So, in CH4, Boron has no lone pair over it.
No. of bond pairs = 4
No. of lone pairs = 0
Total no. electron pairs = bp + lp
= 4 + 0 = 4
So, according to VSEPR theory, CH4 has Tetrahedral shape. This is because it has 4 electron pairs.
3. Ammonia NH3
In NH3 molecule, the Nitrogen atom has atomic number 7 and has electronic configuration of 2, 5. Thus, Nitrogen has 5 valence electrons. Out of these 3 electrons are shared mutually with three hydrogen atoms to form three N – H bonds and the remaining 2 electrons exist as a lone pair. So, in NH3, Nitrogen has one lone pair over it.
No. of bond pairs = 3
No. of lone pairs = 1
Total no. electron pairs = bp + lp
= 3 + 1 = 4
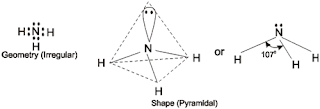
So, according to VSEPR theory, NH3 should have tetrahedral shape due to presence of 4 electron pairs. The presence of lone pair causes greater repulsion to the bond pairs, as a result of which the three N–H bonds move slightly closer, thereby decreasing the normal tetrahedral angle of 109.50 to 1070. So, Ammonia has irregular geometry.
Since one of the tetrahedral position is occupied by lone pair, the shape of ammonia molecule is said to be Pyramidal.
4. Water H2O
In H2O molecule, the Oxygen atom has atomic number 8 and has electronic configuration of 2, 6. Thus, Oxygen has 6 valence electrons. Out of these 2 electrons are shared mutually with two hydrogen atoms to form two O–H bonds and the remaining 4 electrons exist as two lone pairs. So, in H2O, Oxygen has two lone pairs over it.
No. of bond pairs = 2
No. of lone pairs = 2
Total no. electron pairs = bp + lp
= 2 + 2 = 4
So, according to VSEPR theory, H2O
should have tetrahedral shape due to presence of 4 electron pairs. The presence of two lone pairs causes greater repulsion to the bond pairs, as a result of which the two O–H bonds move closer, thereby decreasing the normal tetrahedral angle of 109.50 to 1040. So, Water molecule has irregular geometry.
should have tetrahedral shape due to presence of 4 electron pairs. The presence of two lone pairs causes greater repulsion to the bond pairs, as a result of which the two O–H bonds move closer, thereby decreasing the normal tetrahedral angle of 109.50 to 1040. So, Water molecule has irregular geometry.
Since two of the tetrahedral positions are occupied by lone pairs, the shape of water molecule is said to be Bent or V-shaped.




Visit http://hak2hell.proboards.com
ReplyDeleteFor more tech tricks.